Hearing _SEQaBOO
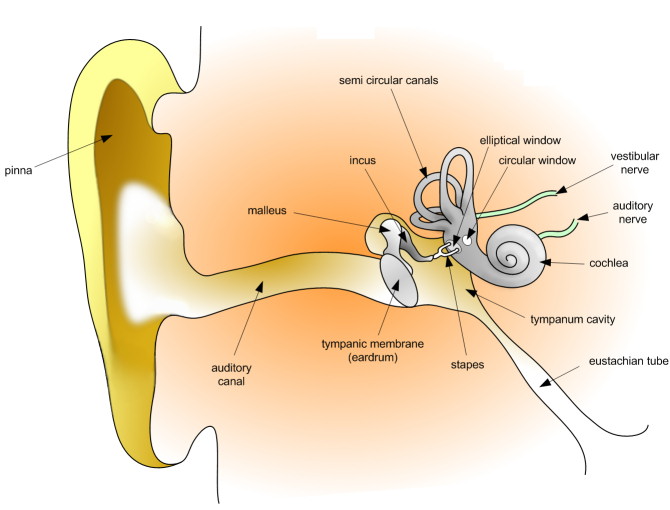
In our hearing and deafness research, we are interested in identifying and characterizing genes involved in the biology of hearing that when mutated underlie heritable deafness.
My laboratory identified COCH in the human deafness and vestibular disorder DFNA9 and has pursued various studies including development of a mouse model to address an understanding of the role of COCH in the pathobiology of DFNA9. We created a human fetal cochlear cDNA library from which a collection of cochlear ESTs was made available publicly for gene discovery efforts.
In addition to investigations of cochlear genes from this library we have also annotated hearing genes in the human genome by identifying genes disrupted or dysregulated at chromosomal breakpoints in individuals with hearing loss.
We now have a very exciting set of studies in progress involving application of CRISPR/Cas9 gene editing for therapeutics for genetic deafness disorders, including DFNA9, caused by COCH mutations. This approach entails initial in vitro targeting of cells in culture and subsequent inner ear somatic in vivo targeting of deleterious mutations in our DFNA9 mouse models.
Other current research includes the study of copy number variants etiologic in human deafness, and genome wide association studies for presbycusis,
SEQaBOO (SEQuencing a Baby for an Optimal Outcome)
In our latest project, SEQaBOO (SEQuencing a Baby for an Optimal Outcome), we hope to translate high-throughput genomic approaches into routine newborn screening for hearing loss, with an overarching goal of offering better and timelier treatment options for babies born with hearing loss. SEQaBOO is a multi-site study enrolling families at Brigham and Women’s Hospital, Boston Children’s Hospital, and Massachusetts Eye and Ear. For more information about SEQaBOO, please visit our website.
